Ella Ceraulo, Innovation Scientist from Cornelius tests the CorFactant range and shares results to aid in the formulation of foam cleaning products.
Foams are integral to cleaning and cosmetics, valued both for their tactile appeal and as a signal of product efficacy. At their heart are surfactants, schizophrenic molecules with opposing hydrophilic heads and hydrophobic tails.1 They effectively lower surface tension, enabling the dispersion, emulsification and generation of bubbles. Surfactant choice not only affects foam richness and texture, but also process efficiency, user experience, and irritation potential.
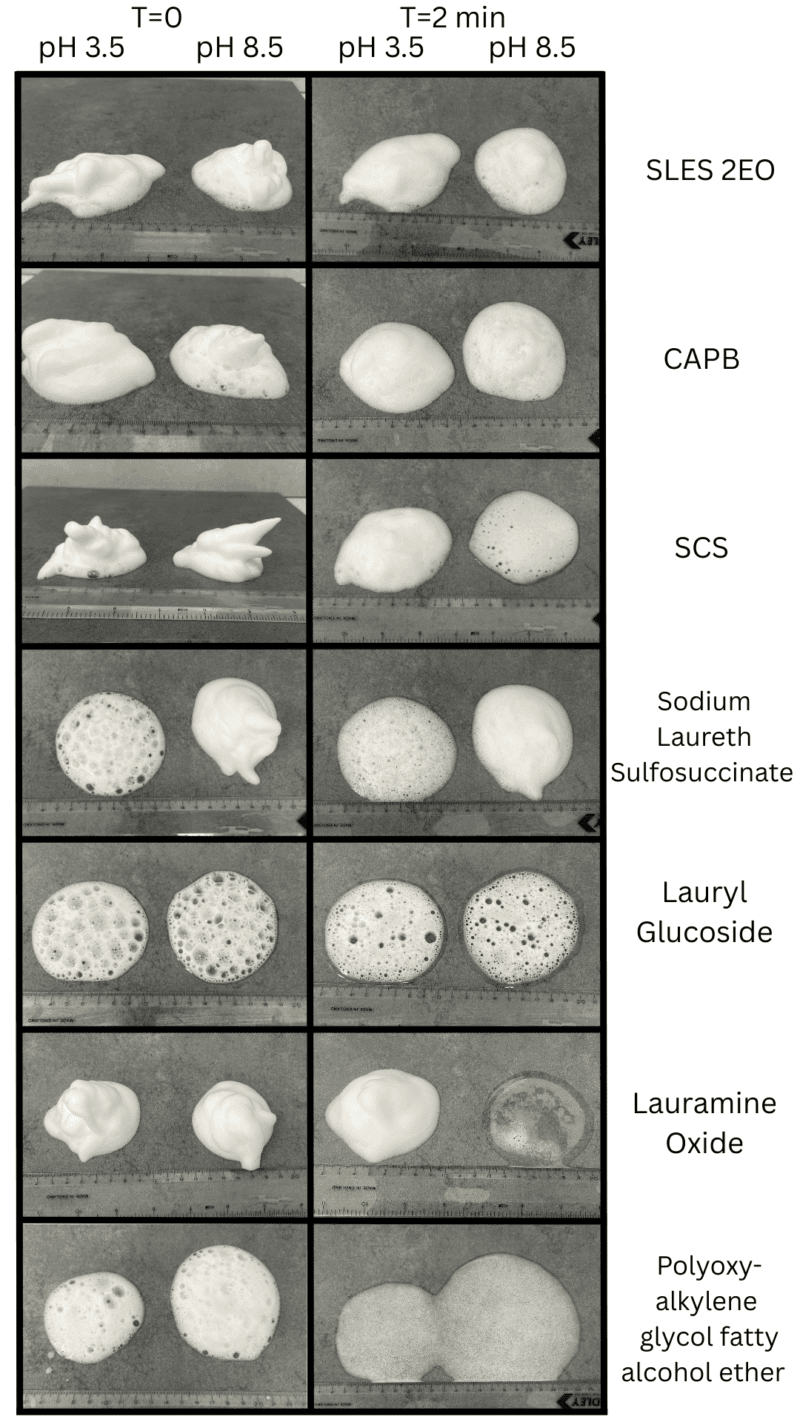
Figure 1 – Foamer pack results
Innovations in foam packaging & delivery
Recent advances in packaging, such as foamer mesh packs and pressure-powered foam generation devices, have enabled richer and more stable foams at the point of use. In these products, foam is produced when surfactant solutions are forced through fine mesh or gauze of 10-50µm, or aerated under controlled conditions, generating a dynamic structure of gas-filled bubbles surrounded by surfactant-laden liquid films. The architecture of these foams—bubble size, uniformity and persistence—is critical for their cleaning power and sensorial qualities.2,3
These formats extend contact time with surfaces, allowing surfactants to act longer on contaminants, softening and lifting dirt and grease more efficiently while minimising manual scrubbing and chemical burden. The sensory quality of foam—its feel, visual appearance and lasting presence—plays a key role in consumer satisfaction where it is a sensory cue for cleanliness. In personal care, where tactile experience directly influences perceived efficacy and product enjoyment, foam structure manipulation has been at the forefront.
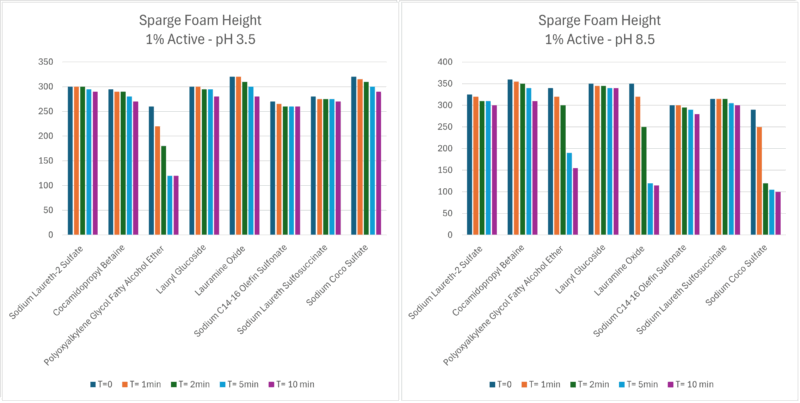
Figure 2 – Sparge foam height results
Taking a cue from the cosmetics industry, we are now seeing household brands differentiating products by encouraging sensorial cleaning rituals. This is often achieved with the introduction of fragrances. However, a new wave of foam surface cleansers offers enhanced efficiency in conjunction with satisfyingly rich foam, possibly enabling foam quality to hold greater appreciation for consumers.
Automotive snow foam cleaning demonstrates these principles: surfactant solutions, delivered via a snowfoam lance with a mesh filter, cling to vehicle surfaces, increasing dwell time, and resulting in measurable water and chemical savings. A Nissan study found a reduction in water consumption by 44%.3
Automotive detailers and car enthusiasts are turning to snowfoam as the preferred wash method for multiple reasons. Firstly, the increased contact time achieved allows the surfactants to soften and lift residues from the surface, reducing the need for labour and time-intensive scrubbing. Secondly, by limiting the abrasive scrubbing action on the car, the risk of causing damage to the lacquer coating is reduced. The benefits of snowfoam can improve efficiency in the transport system, with less downtime required for cleaning time required for buses and lorries.
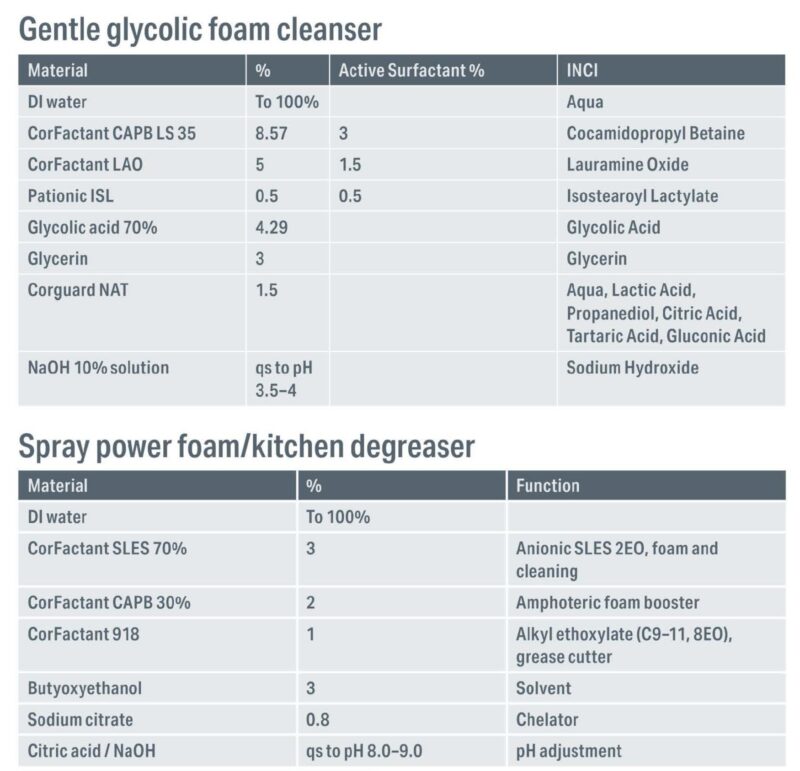
Figure 3 – Formulations with surfactant blends
Surfactant selection: Chemistry & performance
Surfactants fall into four classes: anionic, cationic, nonionic and amphoteric. Each chemical structure offers unique properties for foam formation, stability, and cleaning performance.
Anionics are classified by their negatively charged groups, which can include sulphates and phosphate structures. Alkyl sulfates are stable at a wide pH range, these high foaming surfactants create strong stable foams. Traditional anionic surfactants like sodium lauryl sulfate (SLS) are known for strong cleaning and foaming, but SLS in particular is associated with higher irritation potential and can cause dryness or barrier disruption, especially with repeated use or at higher concentrations.
Sodium cocosulfate (SCS) is a coconut derived milder option than its more commonly used SLS counterpart, which is typically palm derived. They have a wide usage in personal care, industrial, household and automotive cleaning applications.
Alkyl ether sulfates, for example sodium laureth-2 sulfate (SLES) are commonly used in cosmetics, household, such as dish detergents and industrial cleaning applications. Ethoxylation of the alkyl group improves water solubility and increases mildness on the skin, though they can still be drying and interfere with skin barrier function. Higher amounts of ethoxylation can reduce foamability compared to the non-ethoxylated AS counterparts.
Sodium dodecylbenzene sulfonate (SBDS) is used in household cleaning products where it provides a strong, lasting foam. Particularly stable in alkaline conditions, sodium C14-16 olefin sulfonate can offer good hard water stability and biodegradability. High foaming and cleansing ability lend it to household and personal care applications, where it is often used in shampoo formulations.
Sulfosuccinates like sodium laureth sulfosuccinate offer exceptional mildness but with a lower cleaning power than SLES. They are typically used in personal care products aimed at sensitive skin or baby products. Better foaming characteristics are observed at neutral to alkaline pH.
Amphoterics, for example cocamidopropyl betaine (CAPB) or lauryl betaine, charge is pH dependant. In acidic solutions the basic amine group protonates, creating a net positive charge and cationic behaviour.
In neutral pH, they become zwitterionic, while at alkaline pH they behave as an anionic. This flexibility enables them to be used in combination with anionic, cationic and nonionic surfactants where they can boost foam and enhance the mildness of a formulation.
A commonly used amphoteric surfactant, cocamidopropyl betaine (CAPB) is commonly used in personal care applications where it can boost foam, synergistically alter rheology with alkyl ether sulfates and reduce irritation.
Nonionics like lauramine oxide have lower foaming efficacy than anionics but as a secondary surfactant it provides excellent foam boosting properties utilised in personal care and household cleaning.
Alkyl glucosides like lauryl glucoside, exhibit the lowest skin irritation profile. These are widely used in ‘mild’ personal care applications and laundry cleaning products, especially for sensitive skin. They offer mild cleansing, good biodegradability but lower flash foam generation than the alkyl sulfates.
Typically used in combination with other surfactants in personal care products aimed at delicate skin. polyoxyalkylene glycol fatty alcohol ethers (AEOs) are low-foaming surfactants with great cleaning benefits with the ability to ‘wet out’ contaminants. Typically used in household surface cleaning sprays where they offer high pH tolerance. In our testing an AEO was included to demonstrate a surfactant with a lower foam and foam stability characteristic.
Quantifying foam performance
To compare surfactant volume and stability two test were conducted, a laboratory sparge4 and foamer pack dispenser test.4 For both tests 1% active surfactant solutions were prepared in deionised water. The solutions pH was adjusted to pH 3.5 for acidic testing and pH 8.5 for alkaline. This is a much lower inclusion of surfactant than typical of a cleaning product, but was chosen to highlight variations in the surfactants foam creation and stabilisation ability.
In the sparge test, ten seconds of aeration from a bubble stone was applied to a 100 ml test solution. Foam volume and stability were quantified over time, revealing distinct differences in foam strength, persistence, and collapse rates (Figures 1 & 2). The same 1% solutions were then filled into cosmetic type foamer packs and five pumps were dispensed onto a tile for foam observation.
Our testing concluded that the traditional sparge test results aligned closely with the results observed dispensing solutions via a foamer pack and foamer mesh cleaning spray pack. They also confirmed that foam generation and decay dynamics from foam-boosting delivery systems remain highly dependent on surfactant type, concentration and pH.
Results & formulations
Anionic SLES and amphoteric CAPB showed the highest foam volumes and stability, especially in neutral and mildly alkaline conditions. The nonionic lauramine oxide and anionic sodium cocosulfate produced rich initial foam but suffered rapid foam collapse in alkaline systems when used as the sole surfactant. This effect was predicated by the sparge test and visible in the foamer pack results, highlighting the need to considered surfactant selection based on formula requirements and usage conditions.
Based on these results two optimised formulations were created to illustrate how surfactant chemistry can be blended to create performance foam products (Figure 3):
- Spray power foam kitchen degreaser, which uses a blend of high-foaming anionic surfactants with foam boosters designed for alkaline pH. Breaks down baked-on kitchen residues, maximizing persistent coverage and minimising scrubbing, thereby preserving functional coatings on cookware.
- Gentle glycolic foam cleanser, which employs amphoteric and nonionic surfactants balanced for mildness, pH 3.5-5, delivering creamy, stable foam ideal for sensitive skin while maintaining effective cleansing
These formulations, validated by lab testing on foamer and sparge platforms, underscore the strategic selection of surfactant systems for optimal foam quality across cleaning and care applications.
Surfactants’ skin compatibility varies widely, and the drive toward milder, less irritating products has reshaped industry formulation strategies. Nonionics are often the mildest choice for skin, but careful formulation and synergistic blending with higher-foaming surfactants may be necessary in applications where a high-volume flash foam is required. Amphoterics like CAPB are proven to work synergistically with anionics, reducing irritation and sustaining or boosting foam. An anionic/amphoteric blend can be up to 60% milder than pure anionics.
The future
Foam engineering continues to evolve and, in the future, will include systems that can reduce water usage and the chemical burden of cleaning processes. Well-designed foam products, built on scientific understanding of surfactant chemistry and delivery mechanisms, offer compelling benefits for cleaning, sustainability, and user experience, making them central to the next generation of chemical industry solutions. A future emphasis on environmental impact will see the evolution and blending of surfactants to make ever milder but effective products.
References
- P.C. Griffiths, I.A. Fallis, T. Tatchell, L. Bushby & A. Beeby, Advances in Colloid & Interface Science, 2008, 144, 1–2, 13-23, ISSN 0001-8686
- How do foam pumps work: The ultimate guide to it
- Autocar Professional Bureau, 2018, 21, 4
- R. Bois, E. Hecke, I. Pezron, & A. Nesterenko, Journal of Surfactants & Detergents, 2009, 23. 10.1002/jsde.12376